Rime Ice: It’s Cool… Supercool!
2015-02-15 14:31:49.000 – Nate Iannuccillo, Summit Intern
We sure have a lot of rime up here in the winter; in fact, it’s all over the place on the summit. For me a surface coated in rime has always been the classic picture of an extreme Mount Washington winter.
 Rime ice coats the chimney of the Tip Top House.
Rime ice coats the chimney of the Tip Top House.
Ok, so we can see that rime is super cool… but what exactly is rime, and how does it form?
To first present the essential definition, rime can be described as a form of ice that occurs when supercooled water droplets freeze on impact with the surface of an object.
So now we know the definition, but what exactly is supercooled water?
Most people are familiar with the typical freezing point of liquid water as 32⁰ F or 0⁰ C. But did you know that with the right conditions,
water can exist as a liquid well below these temperatures? In fact, liquid water in clouds has been found as cold as -40⁰ F (-40⁰ C), and in a lab setting, liquid water has been cooled to as low as -55⁰F (-48⁰ C)! (Check out
this study performed by the University of Utah in 2011.)
You might be wondering how this is possible…
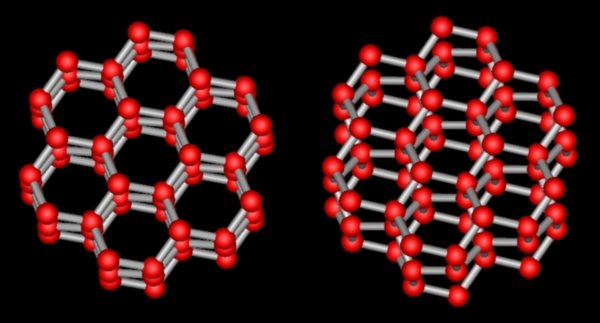
In order to understand this, we need know a little bit about the crystalline structure of ice. When ice forms, water molecules form a fairly rigid crystalline structure called a lattice. To obtain this shape, common ice molecules arrange themselves into a structure of hexagonal boxes that look something like this…
Image courtesy of Kenneth G. Libbrecht, Caltech Dept. of Physics
Alright, getting back to our question about supercooled water…
Let’s think of the crystalline structure of ice as a house cards.
My own house of cards…
Building a house of cards is delicate work, and the first step to any successful construction is finding a suitable surface. Try to imagine constructing your house of cards in thin air without any kind of surface. You couldn’t do it, right?
We can think of ice formation in a similar light. Ice needs some type of surface or motion to initiate the construction of its own structure. In the absence of this surface, water can remain as a liquid below its typical freezing point, becoming supercooled!
Often times, this surface will take the form of what we call ice nuclei. Ice nuclei are tiny particles that allow ice to form around them when coming into contact with liquid water in clouds. There are a wide range of particles that classify as ice nuclei. The bottom line is, without the presence of these particles, supercooled water can be quite common in clouds, as we often see on the summit.
When the wind blows these supercooled water droplets into our observation tower, a trail sign, exposed rock, or any other physical surface, they freeze and crystallize upon impact. For this reason, rime forms in the direction of the wind, and over time can form into the picturesque columns that we frequently see in the winter.
Supercool!
Nate Iannuccillo, Summit Intern
 Rime ice coats the chimney of the Tip Top House.
Rime ice coats the chimney of the Tip Top House.